Regulatory Compliance
GxP Compliance Overview: Ensuring Quality Across Regulated Industries
Author: Gagan Kaur
Mar 26, 2025
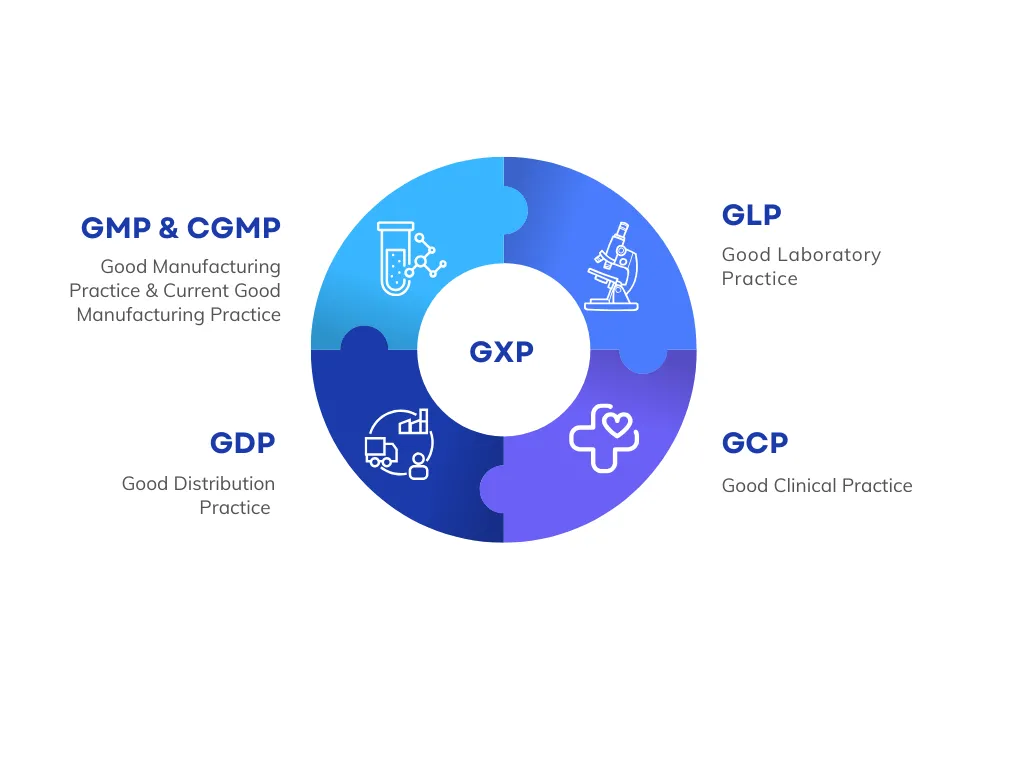
What is GxP Compliance?
GxP compliance is critical in industries where product quality, patient safety, and data integrity are non-negotiable. GxP stands for Good "x" Practices, where "x" refers to specific areas like manufacturing, distribution, laboratory, and clinical processes. Together, these standards ensure that organizations follow validated procedures, maintain proper documentation, and produce reliable, reproducible results.
Industries served by Rees Scientific, such as pharmaceuticals, biotechnology, blood and tissue banks, hospitals, research institutions, and CROs, rely heavily on GxP processes to meet regulatory requirements from the FDA, EMA, WHO, and other global agencies.
What are the Elements of GxP?
Core components of GxP compliance include:
- Data Integrity – Ensuring records are complete, consistent, and accurate.
- Validation and Calibration – Systems and equipment must be validated and routinely calibrated to maintain accuracy.
- Documentation – Every process, deviation, and result must be traceable.
- Quality Assurance and Control – Safeguards are in place to protect product quality and patient safety.
- Regulatory Oversight – Facilities must be audit-ready with documented proof of compliance.
GxP Compliance Made Simple: How GMP, GDP, GLP, and GCP Impact Your Industry
1. Good Manufacturing Practice (GMP and cGMP)
GMP and cGMP (current Good Manufacturing Practice) ensure that products are manufactured consistently with quality standards and regulatory expectations. The "current" in cGMP reminds manufacturers that they must use up-to-date technologies and systems to comply with regulations. This applies to pharmaceutical companies, biotech firms, and medical device manufacturers.
GxP vs. GMP: While GMP is a component of GxP, it specifically focuses on manufacturing, whereas GxP is a broader framework covering multiple compliance areas.
As the life sciences landscape continues to evolve, regulatory authorities emphasize environmental monitoring in pharmaceutical and biotechnology settings. The latest updates focus on:
- Stricter control of environmental conditions such as temperature, humidity, and cleanliness, particularly in cleanrooms and product storage areas.
- Data integrity and reporting, with growing demand for comprehensive, real-time logging systems that can withstand regulatory audits.
- Expanded GMP documentation requirements, including traceability, deviation logs, and audit readiness.
- Enhanced risk management practices, ensuring stable conditions for the safety and efficacy of drug products and biologics.
Facilities that store temperature- and humidity-sensitive materials, such as vaccines, biologics, or gene therapies, must ensure validated, 24/7 environmental monitoring to avoid compliance violations and costly product loss.
Example:
A pharmaceutical manufacturer uses the Rees EMS (Environmental Monitoring System) to constantly track and record the temperature and humidity in cleanrooms. If the environment deviates from validated ranges, alerts are sent instantly to prevent contamination or product loss. Rees Scientific provides GxP calibration for temperature sensors annually to ensure compliance.
Common GMP Environments:
- Biologics manufacturing
- Aseptic processing facilities
- Tissue processing labs
- Sterile compounding pharmacies
2. Good Documentation Practice (GDP)
Good Documentation Practice (GDP) refers to a set of guidelines that ensure all documentation related to pharmaceutical, biotech, and life science processes is accurate, consistent, and compliant with regulatory standards. It is essential for maintaining data integrity, traceability, and accountability throughout a product’s lifecycle.
Why GDP Matters
For regulated industries such as blood banks and biotech companies, precise and compliant documentation is critical. It supports product quality, regulatory compliance, and audit readiness. GDP ensures that all records—whether paper or electronic—are clear, complete, and created in real time.
Example in Practice
A blood bank follows GDP guidelines to document the storage conditions of plasma units, including temperature readings and handling logs.
Rees Scientific supports this effort with automated monitoring and reporting systems that generate secure, time-stamped logs, reducing manual errors and enhancing compliance with regulatory standards.
Common GDP-Relevant Environments
- Pharmaceutical manufacturing and quality control
- Biotech labs and research facilities
- Blood and tissue banks
- Regulated cold storage and monitoring environments
3. Good Laboratory Practice (GLP)
GLP ensures consistency, reliability, and traceability of lab-generated data. Research institutions, biotech companies, and CROs must comply with GLP when conducting non-clinical safety studies. Laboratories must ensure all instruments undergo routine GxP calibration to maintain accuracy in scientific measurements.
Example:
A biotech company developing gene therapies uses the Rees EMS (Environmental Monitoring System) to monitor and record freezer conditions storing cell samples at -80°C. Regular GxP calibration ensures the temperature readings remain accurate, protecting the integrity of their samples and the validity of their study data.
Common GLP Environments:
- Pre-clinical testing labs
- Stability testing labs
- Toxicology studies
- Research universities
4. Good Clinical Practice (GCP)
GCP governs the ethical and scientific integrity of clinical trials, ensuring patient safety and data reliability. This applies to hospitals, CROs, and pharmaceutical companies running trials. Clinical trial facilities must implement GxP process validation to confirm that environmental conditions, such as humidity and temperature, do not impact trial results.
Example:
A Contract Research Organization (CRO) managing a vaccine trial uses Rees EMS to monitor sample storage during the study. Data logging ensures that biological samples are never compromised due to improper storage, meeting GCP requirements. Rees also performs validation of monitoring systems prior to trial start-up to confirm data accuracy.
Common GCP Environments:
- Clinical trial sites
- Hospitals conducting research
- CROs managing trials
- Biotech companies running human studies
What is the Difference Between GxP and GMP?
- GMP is a subset of GxP.
- GxP covers a broader set of standards across different operational areas (manufacturing, distribution, lab, and clinical).
- GMP focuses solely on manufacturing processes and controls.
Why GxP Compliance Matters for Your Operation
Failing to maintain GxP compliance can lead to product loss, regulatory fines, trial delays, or worse—patient harm. Rees Scientific helps protect your operation with:
Partner with Rees Scientific for GxP Compliance
Whether your organization is subject to GMP, GDP, GLP, or GCP compliance, maintaining an effective GxP process is critical for meeting regulatory expectations. Implementing GxP calibration, environmental monitoring, and validation ensures data integrity, product quality, and patient safety.
At Rees Scientific, we help organizations navigate GxP compliance by providing continuous monitoring, calibration, and validation services tailored to your industry’s needs.
Find out how we can help meet your GxP compliance needs. Request and Assessment today.



