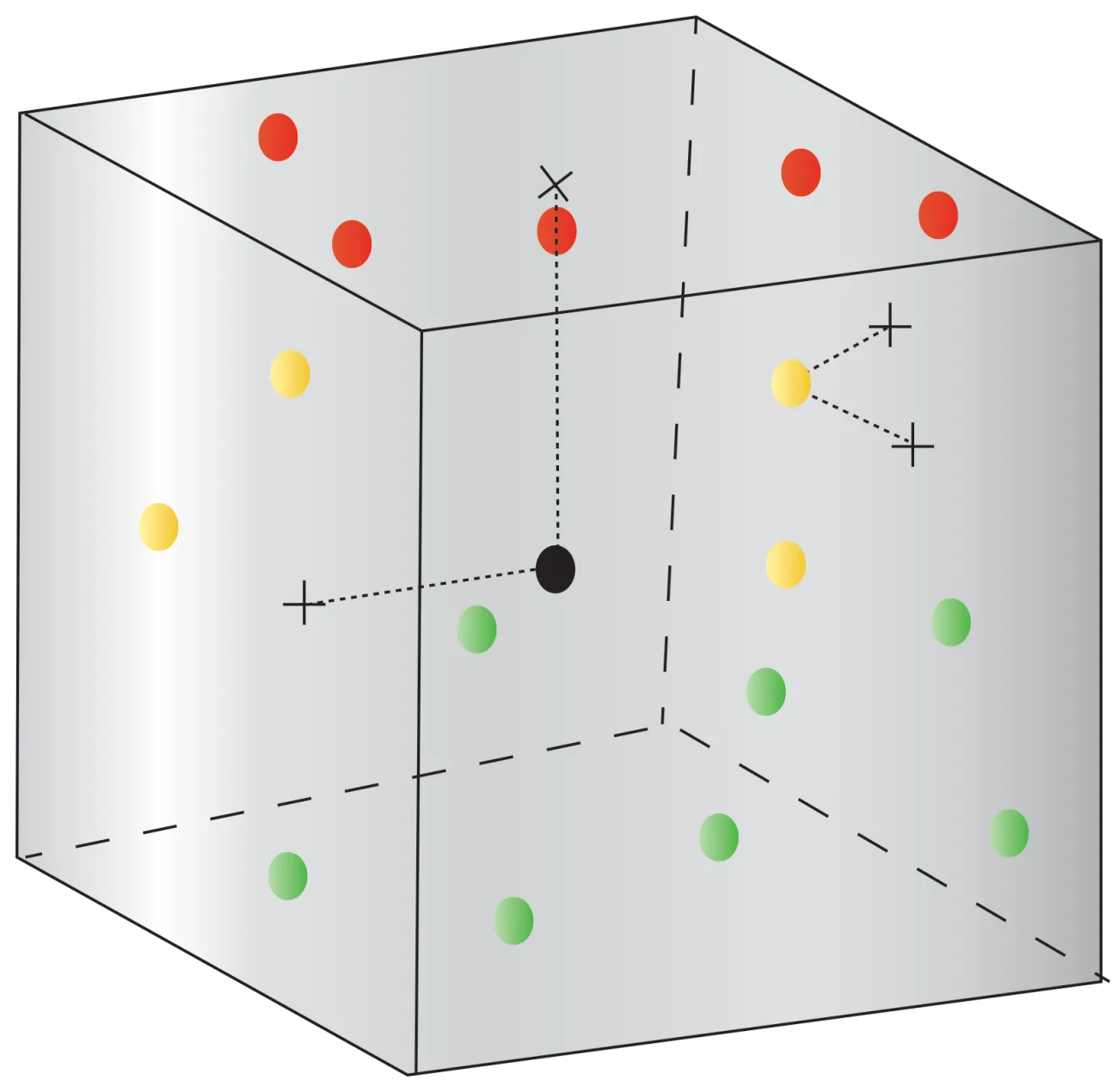
Proper validation of stability chambers, incubators, refrigerators, freezers, and other controlled environments is critical in regulated industries. Mapping temperature and humidity ensures equipment uniformity and identifies any hot or cold spots. Validated mapping provides documented evidence that your chambers meet specified parameters across all zones. This verifies your processes are repeatable and equipment performance is consistent over time. Valid mapping is also required for regulatory compliance, including cGMP, and GLP guidelines. Demonstrating chamber integrity is crucial for audits, inspections, and qualifying for accredited certifications.
Mapping delivers data you can rely on to prove chambers uphold critical specifications. With Rees Scientific's Instant Chamber Mapping kits, you get the validated data you need with extraordinary simplicity, speed, and accuracy. We provide everything you need for fast, reliable validation:
- Multiple wireless sensors to place throughout your chamber
- Connect directly to ReesCloud, PC, or Existing System
- Automated data collection
- Custom reporting with a single click
Simply place the sensors in the equipment, allow them to record data, and easily generate the chamber mapping report through our software. Our comprehensive validation protocol offers kits tailored to diverse needs, ensuring thorough temperature and humidity mapping for general chamber validation. Additionally, we meet more stringent IQ/OQ/PQ qualification requirements with extra sensors, precise calibration, and specialized reports. Collaborating with you, we determine the ideal kit specifications, sensor quantity, placement, and reporting to fulfill your unique validation criteria. With customizable options, we can accommodate any chamber size, shape, or specific requirements.
Instant Chamber Mapping Kits deliver:
- Rees reliability with pre-calibrated, highly stable sensors
- Flexible kits for any size chamber or environment
- Regulatory compliance, including GxP, GLP, and more
- Hassle-free validation across your facilities
Our turnkey kits eliminate the need for manual data logging and complex report generation. With automated sensors and integrated analysis software, validation procedures that once took days or weeks can be completed instantly. The seamless workflows validate chambers promptly and efficiently.
With the automated mapping capabilities of our Instant Chamber Mapping Kits, you can efficiently validate your equipment, meet all regulations, and readily prove data integrity to auditors. During inspections and audits, being able to quickly produce validated reports from our kits demonstrates complete compliance and reliability. All data is automatically logged in secured systems with encrypted audit trails, providing robust documentation that adheres to regulatory audit guidelines. The reports comprehensively display equipment uniformity, highlight any hot/cold spots, and include a full analysis of whether specifications were met across all chamber zones over time. With this validated reporting, auditors can instantly verify your chambers are properly mapped to relevant guidelines and standards.
Our kits provide the objective documented evidence needed to prove controlled environments are within specifications and processes are fully validated. This gives auditors confidence in your compliance and ability to consistently meet critical quality control standards.
Rees Scientific simplifies temperature mapping to expedite your next validation project. We provide economical and tailored solutions to validate equipment and processes throughout your facilities. Our experts collaborate with you to create customized Instant Chamber Mapping Kits that align with your budget and specific validation requirements. Ready to simplify your validation mapping? Contact our experts today to get started with our Instant Chamber Mapping Kits.